Article highlights
-
Erythrasma is a common chronic superficial cutaneous bacterial infection caused by Corynebacterium minutissimum when conditions are conducive.
-
Corynebacterium minutissimum produces coproporphyrin III (a phosphor) that emits a coral pink fluorescence under Wood’s lamp and helps confirm the diagnosis of erythrasma.
-
Smears, cultures and biopsies are only needed in a minority of patients with erythrasma.
-
Erythrasma lesions may be confused with other common skin disorders; identifying the clinical and laboratory features of these conditions helps in the differential diagnosis.
-
Coinfection with fungi or superinfection upon primary dermatosis can occur in erythrasma.
-
The treatment of erythrasma consists of topical and/or oral antibiotics.
-
Re-treatment is effective for recurrent infection; the prevention of erythrasma entails reducing risk factors and using antibacterial soaps.
Erythrasma is a common chronic superficial cutaneous bacterial infection caused by Corynebacterium minutissimum (C. minutissimum), a normal inhabitant of the skin flora. In 1862, the German dermatopathologist Friedrich Wilhelm Felix von Bärensprung coined the name erythrasma to describe this condition and named the organism that caused it Microsporum minutissimum. The nomenclature has undergone metamorphosis several times, and subsequently, the bacteria responsible for erythrasma was reclassified as C. minutissimum. In 1884, Köbner proved its transmissibility by applying the epidermal scales from an individual infected with erythrasma to a healthy person. In 1961, C. minutissimum was isolated from erythrasma lesions for the first time.1
The disease affects predominantly adults but is occasionally reported in children and adolescents.2 Although equally distributed among men and women, erythrasma affecting the groin is more common in men, and interdigital erythrasma is more common in women.3 The estimated prevalence of this condition is between 4% and 15% in the general population, 18% in older adults and 44% in patients with diabetes mellitus (toe web space).4 In a study from Bulgaria, erythrasma was identified in 39% of athletes and 40% of soccer players; both groups had predisposing factors for developing the disease.5 In a study from skin clinics in New Zealand, erythrasma was the most commonly diagnosed infection.6 In two independent studies from Turkey, the prevalence of erythrasma in the web spaces of the toes was over 40%.7,8 A prospective longitudinal observational study from Mexico estimated a prevalence of around 33%.3 A study of military recruits in Denmark yielded a prevalence of approximately 50%.9 Finally, a study from India showed a prevalence of erythrasma of 50% among patients with diabetes and 62.5% among those who are obese.10
Erythrama occurs frequently in obesity and diabetes, two common conditions in internal medicine. Unfortunately, the training curriculum in that speciality does not emphasise this condition. This often leads to misdiagnosis and a protracted clinical course.11,12 The following paragraphs provide a comprehensive review of the topic to educate practitioners and trainees in internal medicine and its subspecialties.
Corynebacterium minutissimum
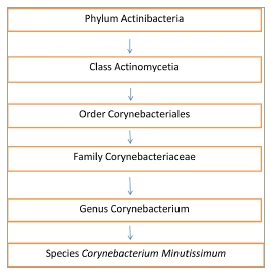
C. minutissimum is a gram-positive, lipophilic, non-capsular, aerobic or facultative anaerobic, non-spore-forming, catalase-producing bacillus presenting as rods or filaments and found as a commensal in the skin (see Figure 1 for taxonomy). A favourable environment permits the bacillus to invade the stratum corneum, where it multiplies, leading to the expansion of this skin layer.1
Figure 1: Taxonomy of Corynebacterium minutissimum, the causative organism of erythrasma
Light microscopy from skin biopsies reveals numerous club-shaped bacilli (Corynebacterium) forming a ‘picket fence’ and mostly occupying the superficial stratum corneum with only a few numbers in the lower part of this layer. Sometimes, a mild perivascular lymphocytic infiltrate is seen.13
The ultrastructure of this bacterium has been defined by electron microscopy. It reveals a thick cell wall that provides a defence or, in some circumstances, the formation of a distinct mucopolysaccharide sheath. The bacterium produces keratolysis at the site of proliferation in the superficial skin; this keratolysis is seen as decreased electron density around the organism and helps spread infection.14 This was the first time that subcellular organelles were demonstrated C. minutissimum by electron microscopy. Well-orchestrated subcellular organelles are a distinctive finding. An elaborate network of mitochondrial enzymes, including nicotinamide adenine dinucleotide phosphate, is a hallmark of enzyme histochemical tests.15 C. minutissimum also has the ability to metabolise various sugars, including glucose.16 This ability may explain its increased occurrence in patients with diabetes. However, the machinery for invading the deeper skin layers is postulated to be absent, explaining the persistence of infection to the skin surface in patients who are immunocompetent. The genome for this organism has been decoded and reveals 2,469 protein-coding regions, 51 transfer RNA genes and 3 ribosomal RNA operons.17 This information may be important for studying antibiotic sensitivity and resistance patterns.
Japanese investigators have isolated other Corynebacterium species from erythrasma lesions (Corynebacterium aurimucosum and Microbacterium oxydans) that produced coproporphyrin III and fluoresced under Wood’s lamp.18
Apart from erythrasma, C. minutissimum causes pitted keratolysis and trichobacteriosis.19 Pitted keratolysis and trichobacteriosis are bacterial infections of the superficial skin and hair, respectively; they are easily distinguished from each other by their distinctive features.19
In hosts who are immunocompromised, C. minutissimum can cause invasive infections such as catheter-related bloodstream infections, prosthetic device and graft infections, peritonitis (in patients receiving peritoneal dialysis), abscesses, postoperative wound infections, osteomyelitis, endocarditis, embolic ocular infections, meningitis, brain abscess, and pyelonephritis.20–30 It is plausible that erythrasma increases the risk for invasive infection in such individuals, although evidence for this is lacking. Rare cases of invasive infections in adults who are immunocompetent have been reported.16
Risk factors of erythrasma
Many risk factors are associated with erythrasma, including hyperhidrosis and obesity, which are conditions that promote a moist environment and skin denudation.10,11 Patients with diabetes or who are immunocompromised are at a higher risk of developing erythrasma as they have diminished defences against the bacterium.12,31 Athletes and sportspersons are also at a higher risk as they are covered with clothing for prolonged periods, and sweat and friction promote bacterial multiplication.5 Overcrowding and poor hygiene in care homes for older adults, hostels, refugee camps, prisons and mental health institutions can promote spread through close contact.32 Living in tropical environments also contributes to multiple risk factors, such as increased sweating, poor hygiene and overcrowding.33
The diagnosis of erythrasma
Clinical picture
Most patients with erythrasma are asymptomatic.1 A few may complain of pruritus, which is usually mild. Mild burning in the affected areas is rarely reported. Yet, others may complain of cosmetically disturbing skin discolouration. In many patients, erythrasma may be discovered by chance while examining the patient for other conditions, such as fungal skin disease and skin lesions of leprosy in tropical countries, or checking for moles.1
Erythrasma is most commonly found in flexural folds: the axilla, submammary, groin, intergluteal, periumbilical, and perianal areas and web spaces between the toes, where the conditions are moist, occluded and macerated by friction.
Erythrasma lesions have been classified as interdigital (toes) or truncal (body areas).1,4 The lesions on the trunk are well demarcated with wavy margins and typically red or brown macules or plaques. The surface of the lesions presents fine scales and may appear wrinkled and sometimes with fissures. Axillary lesions may spread to the inner aspect of the upper arm. In people with darker skin tones, the condition may present as hypopigmented patches with darkened border fissures (Figure 2).4 Extensions and satellite lesions may occur; lesions may coalesce onto neighbouring ones and increase and decrease over time. Normal skin may occur between lesions.34,35
Figure 2: Erythrasma of the axilla
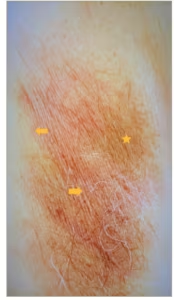
Photo showing reddish patches (arrows), brown hyperpigmented areas (starred) with fine scaly and wrinkled surface. The borders are well demarcated, wavy and extend into the inner aspect of the arm (lower part of photo). Patient received topical fusidic acid with resolution. Photo provided by the author. Patient consent was obtained but deidentified completely.
The skin may get lichenified and develop postinflammatory hyperpigmentation or contact dermatitis from topical therapeutic agents. Web space infection between the toes may present as macerated areas, a red rash or vesiculobullous lesions. They may be painful, itchy or asymptomatic. The physician should separate the toes and examine each web space in good lighting to avoid missing any lesions.7 Atypical presentations are disciform (localized and generalized), subungual, melanotic, vulvar and palmar lesions. Widespread infection may occur.36–40
Various clinical manifestations of C.minutissimum, namely pitted keratolysis, erythrasma and trichobacteriosis, may co-occur in the same patient.41 Coinfection with dermatophytes, other bacteria, and candida has been described.42,43 Clinically persistent lesions after prolonged and repeated courses of topical and/or systemic antifungals should prompt a search for coinfection.
Superinfection upon primary dermatosis, such as psoriasis, hidradenitis, seborrheic dermatitis and pityriasis versicolor, has been recorded. A worsening or increased severity of the primary skin lesions should prompt a search for superinfection.44
Recurrent lesions after initial resolution should prompt a search for diabetes mellitus or HIV infection.
The first step in establishing a diagnosis of erythrasma is establishing the clinical diagnosis. The diagnosis of erythrasma is achieved by maintaining a high index of suspicion in patients with risk factors and clinical characteristics, followed by a Wood’s lamp examination. In most cases, this will suffice for diagnosing erythrasma.
Wood’s lamp
In 1903, Robert Williams Wood developed the Wood’s lamp, a device that uses special filters to block visible light but allow ultraviolet light to pass through. In dermatology, it is used to diagnose several bacterial, fungal, parasitic and pigmentation disorders.45–47 The ultraviolet light induces fluorescence by reacting with pigments (phosphors) produced by an organism. This way, different colours produced by various organisms help distinguish them in a rapid and noninvasive manner. The device is safe to use but will need a dark room with all ambient lights turned off during the brief examination.47
C. minutissimum, the causative organism of erythrasma, produces coproporphyrin III (a phosphor) that emits a coral pink fluorescence with ultraviolet light (Figure 3). This reaction is characteristic of erythrasma and is useful for confirming the diagnosis of the condition, especially in coinfection with tinea, where there is a suboptimal response to antifungals alone. However, the practitioner must ensure that the affected area is not washed or cleaned as the water-soluble coproporphyrin III (and uroporphyrin I) will be washed off, and results will be falsely negative. Some detergents and other substances may cause false positive results.47
Figure 3: Wood’s lamp examination of erythrasma affecting the axillary region

Wood’s lamp examination of erythrasma affecting the axillary region shows coral-pink fluorescence (arrows). Disease extension to the inner aspect of the upper arm is seen in the lower part of the photo. Normal skin has blue colour (arrowhead). Whitish lesions (starred) reflect thickened areas of skin. Photo provided by the author. Patient consent obtained but de-identified completely.
Unfortunately, Wood’s lamp is underused in clinical practice. In India, many primary care providers are unaware of such a diagnostic device, and only a few dermatologists use it in their clinics despite its affordability.
Some authors have suggested using the blue light function in smartphones as an alternative to Wood’s lamp; however, these findings come from a study on vitiligo that did not include individuals with erythrasma.48 As most physicians use smartphones, this would be an attractive alternative to Wood’s lamp. More studies are needed to validate the effectiveness and safety of this method in the diagnosis of erythrasma and other disorders.
Skin biopsy and culture
In cases where the diagnosis of erythrasma is in doubt, as in the case of atypical presentations, skin biopsy with histology and cultures may be required. Such cases are best referred to dermatologists with expertise in this area.
Biopsy and staining of the lesion with Gram stain or special dyes such as methenamine silver, periodic acid–Schiff and methylene blue reveals the organism in the stratum corneum. The diagnosis can be easily missed with ordinary hematoxylin and eosin stains if the pathologist is not provided with the patient history and the disease is not suspected.1
A painless skin surface biopsy technique for examining the stratum corneum has been described.14 This circumvents the disorganization that accompanies the regular biopsy for histopathology.14 A technique that uses an adhesive tape to capture the scales in superficial fungal and bacterial skin infections using a single stain for both organisms has also been described.49 For cultures, tissue culture medium no:199 with sheep blood agar and phenol red has proved optimal.1
In routine clinical practice, these procedures are rarely needed for establishing the diagnosis; however, in the cases studied, a combination of Gram stain and Wood’s lamp has yielded better results than either test alone.
Differential diagnosis
Atypical presentations of erythrasma can be confused with other skin conditions.50–52 At the same time, many common skin conditions can be misdiagnosed as erythrasma. In the following paragraphs, these conditions are discussed, highlighting their distinguishing features.
Intertrigo
Intertrigo is caused by skin maceration due to friction. Many of the risk factors that cause erythrasma play a part in this condition: itching, erythema, maceration, erosions and fissuring. Wood’s lamp tests negative in cases of interigo, and superinfection may occur with candida, fungi and bacteria.53
Candidiasis
Candidiasis also shares common risk factors with erythrasma.53 The presence of erythematous well-demarcated erosive or dry lesions with satellite papules and pustules is helpful in diagnosing this condition. Wood’s lamp is negative in cases of candidiasis. Pseudohyphae, which are commonly found on candidiasis instead of hyphae, are seen on smear and histology.53
Pityriasis versicolor
In pityriasis versicolor, lesions are well demarcated with central hypopigmentation and peripheral scaly hyperpigmented macules.50 Skin scraping potassium hydroxide preparation test of this condition reveals spores and hyphae, and histology specimens reveal a ‘meatball and spaghetti’ morphology. A orange-yellow fluorescence with Wood’s lamp is indicative of the presence of pityriasis versicolor.50
Tinea
Tinea is a fungal infection characterized by itching and erythematous scaly lesions with a raised geographic border.54 It can affect the feet (tinea pedis), the groin (tinea cruris) and the top layer of the skin throughout the body (tinea corporis). In cases of tinea, Wood’s lamp is negative, although it detects green fluorescence in cases of microspora infection. Finally, potassium hydroxide smear tests are positive, revealing hyphae with septae.54
Acanthosis nigricans
Acanthosis nigricans presents as dark, velvety, non-scaly hyperpigmented lesions in the folds (both in flexure and extensor areas). 55These lesions do not have erythema or annular borders. The condition is often accompanied by obesity and a personal and/or family history of diabetes. In cases of acanthosis nigricans, Wood’s lamp and potassium hydroxide smear tests result as negative, while histopathology shows characteristic findings and absent organisms.55
Contact dermatitis
Contact dermatitis can occur in skin folds. Lesions are erythematous but often not well defined.4 A careful history may reveal the use of irritants such as perfumes, and patch testing will help confirm the diagnosis. In cases of contact dermatitis, Wood’s lamp examination is negative.4
Inverse (flexural) psoriasis
Inverse psoriasis, also known as flexural psoriasis, is a variety of psoriasis occurring in the skin folds.56,57 It is more inflammatory than plaque psoriasis, the most common form of psoriasis, and does not have the characteristic appearance of its traditional counterpart. It may occur along with conventional psoriasis or per ipsum. The lesions are erythematous plaques, and the lesion sites are similar to erythrasma. However, intense itching and a younger age of onset – it is more common in children – help distinguish the two conditions. Furthermore, these lesions do not fluoresce with Wood’s lamp, unlike erythrasma. Nonetheless, superinfection of the disease with fungi and bacteria, including C. minutissimum, may complicate the picture. A primary bacterial infection is postulated to trigger inverse psoriasis; however, it is still unclear whether erythrasma triggers an immune process resulting in psoriasis or, vice versa, it is psoriasis that leads to erythrasma.56,57
Seborrheic dermatitis
Seborrheic dermatitis is characterized by erythema, erosions, fissures and yellow crusts.58 Apart from flexural folds, the face and scalp are characteristic sites of this condition. In cases of seborrheic dermatitis, Wood’s lamp is negative.58
Treatment
There is no consensus about which compound should be used as the initial treatment of erythrasma. The choice of the initial treatment depends on patient factors as well as the physician’s preference. The cost of therapy and compliance are also important considerations. In general, oral medications are preferable for treating extensive lesions, and topical medications are preferable for smaller lesions.59–61 In some cases, such as in noncompliant patients, a combination of the two modalities may be necessary. However, no real-world studies are available to support this approach. Few studies compare the use of these treatment regimens in erythrasma.62,63 In the past, azole antifungals have been used but are currently not a first-line treatment; however, they are useful when erythrasma is coinfected with fungi.64 Table 1 lists therapies commonly used to treat erythrasma and important considerations for the individual medications.
Table 1:Common treatment options for erythrasma
|
Topical therapy |
Considerations |
|
Fusidic acid |
2% ointment 2 weeks treatment Minimal side effects Resistance documented Novel drug delivery routes available:
|
|
Clindamycin |
2% lotion or ointment 2 weeks treatment Minimal local side effects No systemic toxicity Resistance reported |
|
Mupirocin |
2% ointment 2 weeks treatment Minimal local side effects No systemic side effects |
|
Whitfield ointment |
Benzoic acid 6% plus salicylic acid 3% in a petrolatum base Twice daily application for 2 weeks Works mainly by keratolytic effects rather than antibacterial Similar efficacy to systemic erythromycin for axillary or groin lesions Superior to oral agents for interdigital lesions Irritation is a minor side effect |
|
Benzoyl peroxide |
Highly reactive oxygen radicals destroy bacteria No formation of bacterial resistance Component of antibacterial soaps |
|
Oral therapy |
|
|
Erythromycin |
250 mg QID for 14 days Minor GI side effects common QTc prolongation: caution with p 450 inhibitors |
|
Clarithromycin |
1 g single dose Side effects similar to erythromycin |
|
Tetracyclines |
250 mg QID for 14 days Minimal GI side effects Photosensitivity |
GI = gastrointestinal; QID = four times a day.
Antibiotic resistance
Widespread antibiotic use encourages the development of resistant bacterial strains. This is also true in the case of C. minutissimum. Many studies have shown increasing resistance of this bacteria to macrolides, which are the mainstay of therapy against bacterial infection.65,66 Bacterial genes that promote resistance have been mapped. They are detected by polymerase chain reaction and may guide the choice of therapy in the future. The percentage of patients resistant to various antibiotics varies among different geographic areas and may reflect patterns of use and testing methods. Antibiotic stewardship for use in erythrasma should be in place.66
Other novel treatment modalities
In the era of ever-increasing antibiotic drug resistance, some novel treatments that do not share this problem may find a place in the therapeutic armamentarium of erythrasma.
Phototherapy
Red light activates the pigments produced by C. minutissimum, and the resulting photodynamic activation damages the bacteria, causing cell death. In a pioneering study, Darras-Vercambre et al. treated 13 patients with erythrasma with one or two illuminations of red light and found a complete cure in 3 patients and at least a 30% reduction in the remainder.67
Zeina et al. used antimicrobial photodynamic therapy on the skin utilizing methylene blue and visible light and studied the effects of light intensity in killing various organisms.68 The list included C. minutissimum, which portrayed a D value of 120 s. A similar response was seen with natural sunlight.68
Local antiseptics
Nishioka et al. examined the antibacterial efficacy of olanexidine compared with chlorhexidine and povidone-iodine, two well-known antiseptic agents, on an ex vivo porcine skin model and had good results.69 If found to be successful in large human trials, this novel method may help prevent recurrences.
Ozonized olive oil
Ramírez-Hobak et al. studied the local application of ozonized olive oil twice daily for 10 days on 10 patients and found a similar response to oral erythromycin.33 The ozone produces bactericidal action via reactive oxygen radicals delivered to bacteria. Drug resistance was not reported. This therapy is yet to be validated in larger trials.
Drug delivery systems
Fusidic acid-based topical liposomal gel formulations enhance drug delivery to the skin. Fusidic acid-based niosomal drug delivery systems are more stable than liposomal-based systems. Niosomes are nonionic surfactants and multi-lamellar vesicles created to act as a local drug reservoir that ensures steady, constant volume delivery into the stratum corneum.70 Furthermore, a polymeric nanoparticle preparation of erythromycin stearate with improved absorption and availability has shown promise.71
Complementary and alternative medicines
A number of plant-based compounds used in indigenous systems of medicine are reported to be effective in treating several dermatological conditions, including erythrasma.72,73 More robust trials are needed to define their role as treatments for this condition.
Prevention of recurrence
To prevent the recurrence of erythrasma, the treated areas must be kept clean by regular washing and implementing other hygiene measures, such as avoiding skin-to-skin contact; not sharing towels, razors, soap or clothes; and not touching wash basin faucets and disposable wipes. Excessive sweating must be prevented with appropriate sweat-absorbing clothing, fans, air conditioning or sweat absorbers. Weight loss, control of diabetes and correction of immunosuppression are other important measures in appropriate patients, such as weight loss in patients who are obese and blood glucose control for those with diabetes. Periodic and meticulous surveillance for the recurrence of erythrasma by patients and physicians is also necessary. The use of antibacterial soaps and early treatment of recurrences are also recommended.
Future directions
A better understanding of the infective process, spread and mechanisms underlying the recurrence of erythrasma and antibiotic resistance will help improve outcomes in erythrasma. The differences in the behaviour of the organism between hosts who are immunocompromised and those who are not immunocompromised will prove resourceful in patients with HIV and for those receiving cytotoxic chemotherapy. Moreover, the novel treatment modalities for the disease discussed earlier need further validation. Finally, practice guidelines for the diagnosis and management of erythrasma and curriculum changes need to be urgently developed to educate internists and subspecialists to enable them to identify and manage this condition.