The Russian invasion of Ukraine represents the largest humanitarian crisis that Europe has faced in decades. In the first 4 months of the war, over 9.9 million refugees crossed the Ukrainian border, and over 6.1 million Ukrainian refugees were registered across Europe.1 Before the war, Ukraine had a high prevalence of tuberculosis (TB); the prevalence of latent infections with M. tuberculosis was extrapolated based on the incidence rate to be up to 20%.2 Moreover, Ukraine has high rates of HIV and hepatitis. Specifically, 260,000 individuals in Ukraine were infected with HIV.3 In 2020, 40.4% of intravenous (IV) drug users in Ukraine were infected with the hepatitis C virus (HCV).4 The disease burden is further increased by coinfections. Ukraine has the second-highest rates of HIV/TB coinfection in Europe and high rates of multidrug-resistant TB (MDR-TB)/rifampicin-resistant TB (RR-TB), representing 31% of bacteriologically confirmed new TB cases in Ukraine in 2021.5–7 Ukraine reports a high rate of antibiotic-resistant bacterial isolates8 and low vaccination coverage,9,10 including for coronavirus disease 2019 (COVID-19), whose vaccination rate as of 27 February 2022 was 39.7%.11 The military conflict had a major impact on the control of COVID-19 in Ukraine.12
TB remains the leading cause of death by a bacterial pathogen, and it resulted in 1.6 million deaths worldwide in 2022.7 Antimicrobial resistance against isoniazid and rifampicin, the two most efficient medicines in the standard anti-TB regimen, has been emerging with proportions above 20% in Eastern Europe.7 Classifications of drug-resistant TB have recently been revised by the World Health Organization (WHO).13 RR-TB is accepted as an approximation for MDR-TB, which together is termed MDR/RR-TB, and was responsible for an estimated 450,000 new cases worldwide in 2021.7 Pre-extensively drug-resistant TB is defined as MDR/RR-TB plus resistance to a later generation fluoroquinolone, such as levofloxacin (Lfx) and/or moxifloxacin (Mfx). Extensively drug-resistant TB is defined as when pre-extensively drug-resistant strains of M. tuberculosis exhibit additional drug resistance to linezolid (Lzd) and/or bedaquiline (Bdq).14 The war in Ukraine will probably result in severe drawbacks in the fight against TB in Eastern Europe because of the destruction of hospitals and infrastructure, suspension of specialized patient care (causing delayed or suspended treatment), worsening or reactivation of the disease due to overcrowding and malnutrition, and increased transmission rates among displaced persons, particularly children.15,16
While the European and National Health Authorities have published guidelines for screening and priorities for medical care among Ukrainian refugees,17 the diagnosis and treatment of individual patients from Ukraine can still be challenging. Here, we present the case of a patient with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), HIV and HCV infection, whose treatment of MDR-TB proved to be clinically challenging.
Compliance with ethics
The patient agreed to the publication of his medical history and images. Ethics approval is not required for case reports because of a waiver for case reports with given informed consent of the patient. All medical and scientific actions were performed in accordance with the Helsinki Declaration of 1975 and its later amendments. Our patient gave his written informed consent to publication. He signed a consent form for publication on 24 May 2022. The first author, Kristina Russu, translated the form into Russian. We omitted all identifiable pieces of information.
Case Report
In April 2022, a 53-year-old male patient was hospitalized in our infectious diseases department. He was a Syrian citizen who emigrated to Ukraine approximately 20 years ago. One day prior to hospitalization, he reached Germany after fleeing the Russian troops who invaded the territory of Ukraine.
In 2018 and 2019, he received full treatment for TB and was hospitalized for 5 months. The patient reported that he received treatment for drug-sensitive TB. However, as the patient had not recovered, the treating physicians assumed that it was drug-resistant TB. He had a large cavity involving the upper and middle lobe of the right lung as a sequela of TB. Moreover, severe leg pain owing to neuropathy has persisted since TB treatment in 2019.
Additionally, he was diagnosed with chronic HIV infection in 2019. Antiretroviral therapy (ART) with dolutegravir, lamivudine and tenofovir disoproxil (DTG/3TC/TDF) was initialized. No further information about viral load and CD4 cell count and adherence was available.
Upon arrival in Germany, the patient complained of abrupt onset of dyspnoea and severe coughing, which had begun as he fled Ukraine. A chest X-ray performed the day after his arrival in Germany showed structural abnormalities in the right lung, and he was referred for further examinations. The physical examination showed no overt pathological findings and, in particular, no pathological signs in the lungs, and we saw no sign of liver cirrhosis. A computed tomography (CT) scan confirmed the X-ray findings of abnormalities in the right lung (Figure 1).
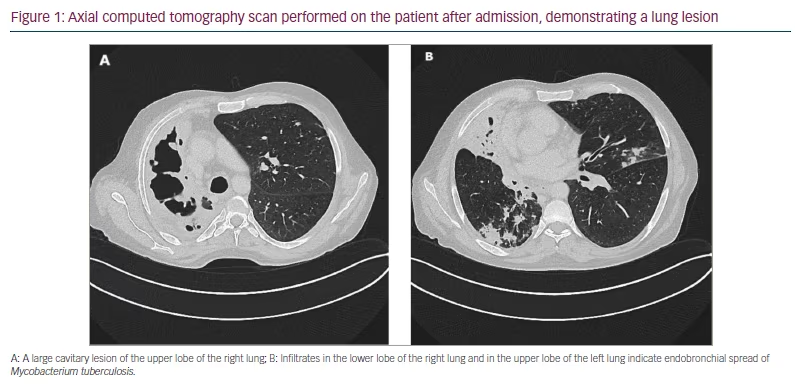
SARS-CoV-2 reverse transcription-polymerase chain reaction testing on the day of admission was positive (viral load <10,000 copies per mL). As an unvaccinated patient with COVID-19 and chronic lung disease, he received remdesivir intravenously for 3 consecutive days (200 mg once per day [OD] on day 1 and 100 mg OD on days 2 and 3). He tested negative for SARS-CoV-2 after 1 week.
During fleeing, ART was available, and his HIV viral load was below the limit of detection. The CD4 T cell count was 192 cells/µL. HCV infection was newly diagnosed two days after admission by the presence of anti-HCV antibodies and HCV RNA (viral load 776,374 IU/mL). Hepatitis B virus (HBV) was not detected. Liver cirrhosis with splenomegaly was confirmed by a CT scan. However, it was not in an advanced, decompensated state; liver function and coagulation function were in a normal range and remained normal throughout the observation time.
Microscopic examination of sputum on the day of admission revealed acid-fast bacilli (grading 1+ according to Centers for Disease Control and Prevention guidelines). The presence of M. tuberculosis complex bacteria was detected by automated GeneXpert® (Cepheid, Sunnyvale, CA, USA) nucleic acid amplification on sputum specimens. Amplification of part of the bacterial ribosomal B gene showed that the base-pair sequence was not identical to the wild-type sequence, suggesting rifampicin resistance. Identification of M. tuberculosis in culture and drug susceptibility testing took a total of 14 days before we were able to start the administration of anti-TB medicines. The first-line drug susceptibility testing revealed sensitivity against fluoroquinolones and amikacin (Am), while rifampicin and isoniazid were resistant. Resistance to isoniazid was based on a mutation in the M. tuberculosis catalase-peroxidase enzyme [katG] gene. M. tuberculosis isolates were sent to the National Research Center Borstel–Leibniz Lung Center for phenotypic second-line drug susceptibility testing and genotypic prediction of drug susceptibility or resistance. Anti-TB therapy was initiated with Mfx (400 mg OD oral), Bdq (400 mg twice a day [BID] oral), Lzd (600 mg OD oral), terizidone (Trd) (750 mg OD oral) and clofazimine (Cfz) (planned 100 mg OD oral, not received before final resistance testing). Later we received the final results of the resistance testing (Table 1).
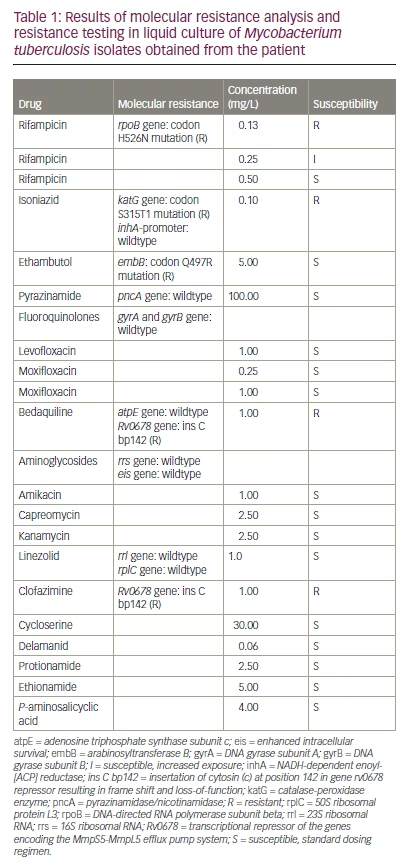
As M. tuberculosis was shown to be resistant against Bdq and Cfz on the basis of a mutation in the Rv0678 gene (transcriptional repressor of the genes encoding the MmpS5-MmpL5 efflux pump system), the therapy was changed to Mfx (400 mg OD oral), Lzd (600 mg OD oral), Trd (750 mg OD oral), Am (500 mg OD IV), meropenem (Mpm) (2 g three times a day [TID] IV), Amoxicillin/clavulanic acid (875 mg/125 mg TID oral) and delamanid (Dlm) (100 mg BID oral). The mutation in the DNA-directed RNA polymerase subunit beta (rpoB) gene leading to rifampicin resistance turned out to be a rare variant (H526N), which leads to lower levels of drug resistance than the most common mutation in S531L.18 ART was changed to a one-tablet regimen consisting of bictegravir (50 mg), tenofovir alafenamide (25 mg) and emtricitabine (200 mg) to reduce potential drug-associated nephrotoxic side effects of TDF, in case of co-administration of nephrotoxic drugs such as Am.
Directly before starting adequate TB therapy, a bacterial superinfection of the lung cavity was suspected (day 20 after admission). Klebsiella pneumoniae, which produces the carbapenemases oxacillinase-48 and New-Delhi metallo beta-lactamase (NDM-1) and is only susceptible to high doses of Mpm, was cultured from the respiratory specimen. Serology for Aspergillus and cryptococcus was negative.
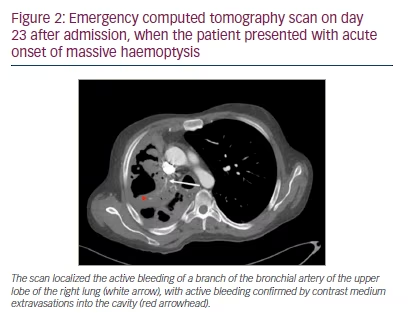
On day 23 after admission, the patient presented with acute onset of massive haemoptysis. After stabilization with vasoactive drugs (terlipressin 1 mg to 9 mL NaCl 0.9% per inhalation), cyclocaprone (1 g BID IV for 14 days) and blood transfusion, emergency CT scan and angiography showed an active haemorrhage originating from a branch of the bronchial artery of the upper lobe into the cavity (Figures 2 and 3).
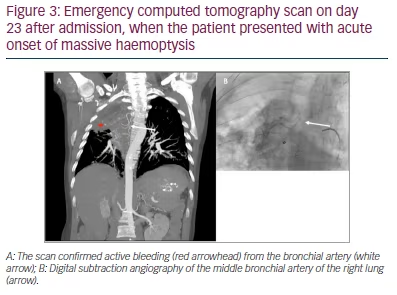
Treatment involved embolization with calibrated microparticles (400 µm) and microcoiling of the right bronchial artery. Angiography of the right pulmonary artery revealed no pathology. After the intervention, no further active bleeding was observed.
In due course, the regimen had to be modified due to side effects. Am was stopped because of high-frequency hearing loss. Mfx was changed to Lfx due to elevated laboratory parameters of cholestasis. High-dose Mpm was reduced. The treatment consisted of Mpm (1g TID IV)/Amoxicillin/clavulanic acid (875 mg/125 mg TID oral), Lfx (1000 mg OD oral), Trd (750 mg od oral), Lzd (600 mg OD oral) and Dlm (100 mg bd oral). After 9 months of treatment, isolation could finally be suspended as no cultural growth of mycobacteria was seen, although sputum examinations still demonstrated acid-fast bacilli on microscopy. These bacilli were not culturable and, therefore, not infectious. The patient, however, needed longer hospital care due to long-term oxygen supplementation and the requirement of IV antibiotics.
Discussion
In this case report, we presented a Ukrainian patient with MDR-TB and HIV/HCV coinfection, concomitant bacterial superinfection with carbapenemase-resistant Klebsiella pneumoniae, and complicated management of a life-threatening pulmonary haemorrhage by bronchial artery embolization.
Our case illustrates the vulnerabilities to infectious diseases that refugees fleeing Ukraine face and the need for awareness and syndromic surveillance of these diseases in countries welcoming refugees. According to operational public health considerations by the European Centre for Disease Prevention and Control, structured syndromic surveillance should include HIV/TB coinfection, MDR-TB, HBV/HCV coinfection, SARS-CoV-2 and antimicrobial resistance, as well as measles and polio.17 However, in our opinion, the prevalence of these diseases does not justify the general screening of all refugees, and the risk of a high influx of transmissible diseases into neighbouring countries is low. The European Centre for Disease Prevention and Control and WHO recommend specific testing of at-risk populations and contacts.19 Updated information on prevalence is needed in this on-going humanitarian crisis. Medical societies such as the German AIDS Society (DAIG), the German Working Group of HIV practitioners (dagnä) and Paediatric Working Group AIDS (PAAD) provide care and supervision for specialized care of displaced persons.
Treatment of MDR-TB in a patient with chronic HIV and HCV infection proved to be challenging in many ways and required meticulous precautions to protect the healthcare personnel involved from contracting MDR-TB. For treatment of MDR/RR-TB, WHO recommends second-line drugs, categorized into three groups based on available data of effectiveness:
-
group A (Lfx/Mfx, Bdq, Lzd)
-
group B (Cfz, cycloserine or Trd)
-
group C (ethambutol [Emb], Dlm, pyrazinamide [Z], imipenem-cilastatin or Mpm, Am or streptomycin S, ethionamide [Eto] or prothionamide, P-aminosalicyclic acid).20
Two main regimens are recommended by the WHO:
-
A shorter all-oral regimen containing Bdq (6 months), Lfx/Mfx, Cfz, Eto, Emb, high-dose isoniazid and Z for 4–6 months, followed by Lfx/Mfx, Cfz, Emb and Z for an additional 5 months
-
Longer regimens (18 months) containing Bdq (6 months or longer), Lfx/MfX, Lzd, Cfz or cycloserine/Trd.
Additional group C drugs (Dlm, Emb, Z, Am, Eto, P-aminosalicyclic acid or clavulanic acid) may be included.20 In a rapid communication released in May 2022, the WHO recommended a short regimen containing Bdq, Pretonamid, Lzd (BPaL) (600 mg per day) and Mfx for 6 months (the BPaLM regimen).21 However, short regimens such as BPaL have specific requirements and contraindications (e.g. additional resistance) and cannot be recommended as the preferred regimen for all MDR/RR-TB patients in Europe. In addition, the BPaLM regimen does not have the approval of the European Medicines Agency yet.22 Individuals chronically infected with HIV are highly vulnerable to reactivation and severe course of TB. Moreover, the outcome of MDR/RR-TB is worse in patients with HIV;23 however, the risk of death can be significantly decreased by ART and second-line drugs for MDR/RR-TB.23 ART should be started as early as possible, specifically no later than 2 weeks after initiation of anti-TB therapy, except for TB meningitis.22 The interaction of ART with anti-TB drugs has to be carefully evaluated. Disease course or drug therapy can be further complicated by coinfection with HBV and HCV. While antiviral treatment against HBV can be included in ART by choosing a TDF-containing regimen, HCV therapy requires a 2–3 month treatment course of directly acting antivirals (DAAs). HCV treatment should be started after establishing stable anti-TB therapy and ART. HCV treatment is contraindicated with rifampicin-containing regimens and must be postponed. For second-line drugs for MDR/RR-TB and DAAs, no major interactions are to be expected, but data are scarce. Only a small retrospective, observational cohort study showed that MDR/RR-TB treatment in HCV-positive individuals was effective and well tolerated.24 Therefore, HCV treatment should be considered to prevent TB treatment-associated hepatic side effects and consecutive treatment interruptions. Unfortunately, owing to the lack of reimbursement of DAA therapy in Germany via the diagnostic-related group healthcare system, patients who are HCV positive do not receive therapy while hospitalized; this represents a considerable delay for patients with long hospital stays, such as patients with TB. Therapy of relapse is even more difficult in the face of already established sequelae of the former therapy, such as neuropathic pain in our patient and risk of liver decompensation and hearing loss in the further course.
Haemoptysis can complicate the course even more. Hygienic precautions regarding splashes of blood from patients with MDR-TB/HIV/HCV coinfection are important. Following airway management, selective angiographic examination and bronchial artery embolization in specialized centres is a safe and effective treatment for massive haemoptysis, as surgical options are often limited due to excessive bleeding and the physical condition of the patients.25 In case of failure of embolization, surgical resection should be considered.25
The complex management of our patient was even more challenging due to the impact of the humanitarian crisis. Communication and provision of psychological care to refugees are particularly challenging in a hospital setting because isolation of the patients is often necessary for weeks or even months to minimize the risk of transmission. After the patient fled Ukraine, data on his medical history and imaging were often not available. Without his medical history, the treatment for MDR-TB of our patient was on hold for weeks, until the results of resistance testing were able to guide the assembly of an effective drug regimen. We could not clarify whether our patient already had drug-resistant TB in 2018 and 2019 and if treatment had been successfully completed. The unusual drug resistance pattern shown may indicate suboptimal treatment, resulting in relapse while he was fleeing Ukraine. In Ukraine, success rates of treatment, although for the most part available, were already suboptimal before the war, that is, at about 50%.2,5 In total, our case exemplifies the high importance of exchanging health data. The WHO and the Ukrainian health centres have established a system of data sharing for providing the medical history of patients with HIV.26 Of course, data protection measures have to be carefully established in this vulnerable group. Therefore, data sharing is restricted to health providers who obtain signed informed consent from the patient. Further potential delays may be connected to the availability of salvage drugs; for example, Cfz needs to be imported to Germany. Other salvage drug stocks needed to be piled up due to the rarity of MDR-TB before the crisis. Specifically, Bdq and Cfz are cornerstones of MDR-TB therapy, and additional drugs such as Dlm (group C) may be important as well. In general, the healthcare systems seem well adapted and equipped for the health crisis, and the additional TB cases have remained low so far. However, increasing numbers of hard-to-treat TB following treatment interruption, reactivation due to undernutrition and stress during fleeing, and lack of access owing to language disparities and lack of familiarity with the healthcare system of host countries are possible challenges.
Limitations of this case study are the lack of data on the patient’s history and, most importantly, resistance testing results and the administered regimen of the first TB episode. This could have been revealed if the TB had been reactivated.
Conclusion
This case illustrates the specific infectious disease vulnerabilities connected with the humanitarian crisis resulting from the Russian attack on Ukraine. Complex management of refugees with several coinfections can be challenging and requires updated information on epidemiology, protected exchange of medical data, and well-adapted healthcare systems, including infectious diseases expertise and psychological care.



