With the on-going coronavirus disease 2019 (COVID-19) pandemic challenging almost every aspect of life, immunity to COVID-19 following vaccination or infection has become the centre of attention. Several vaccines provide protection, but the duration of protective immunity is not known. Even less understood are the duration of protection after natural infection and the role of different immune cells.1 Specific interest has been given to patients with impaired immune function due to primary immunodeficiencies, HIV, immunosuppressive medication or immunosenescence. Here we present the disease course of a patient with X-linked agammaglobulinaemia (XLA) following infection and confirmed reinfection after 1 year with different SARS-CoV-2 virus strains before the onset of the omicron wave.
Case report
This 30-year-old male patient with XLA had a history of multiple infectious diseases. He was receiving weekly subcutaneous immunoglobulins (SCIG). In April 2020, he presented with a 10-day history of fever and abdominal pain, dyspnoea and shortness of breath. His respiratory rate was 20 breaths/minute, and he had an oxygen saturation of 90% on room air. The SARS-CoV-2 infection was confirmed by polymerase chain reaction (PCR) in an oropharyngeal swab (see Supplemental Table 1 for the methods and kits). He received oxygen supplementation via a nasal cannula.
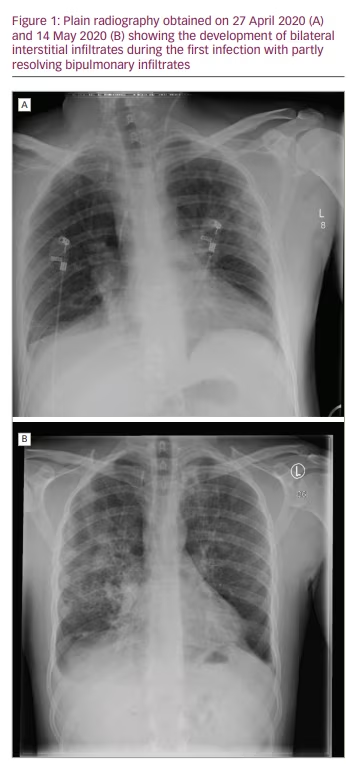
Initially, C-reactive protein (CRP) was 64.7 mg/L (normal values: <5.0 mg/L) and peaked at 170 mg/L (day 10). Leukocytes and lymphocytes were normal, and platelet count was 91 × 109/L (normal value: 150–370 × 109/L), with a minimum of 81 × 109/L (day 2). Immunoglobulins were tested once, and a value of 3.99 g/L (normal values: 7.0–16.0 g/L) was found during peak inflammation. Chest X-ray initially showed no signs of pneumonia (Figure 1A) but did later in the disease course (Figure 1B).
The administration of convalescent plasma was discussed but dismissed due to only mild symptoms at that time and fear of severe inflammation via an antibody-enhanced inflammatory response, which would worsen the clinical status. Since no specific anti-viral medication was available, the patient received intravenous fluids, prophylactic
low-molecular-weight heparin and anti-pyretics.
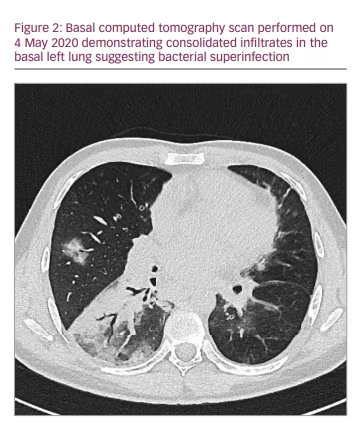
A computed tomography scan showed ground-glass opacification and infiltrates assigned to bacterial superinfection (Figure 2). After defervescence and ceasing supplemental oxygen on day 19, he could be discharged on day 23. He was symptom free and tested negative for SARS-CoV-2 via PCR multiple times. Enzyme-linked immunosorbent assays (ELISAs) for anti-SARS-CoV-2 antibodies proved inconclusive at the time of discharge. One ELISA showed signs of immunoglobulin (Ig) G, and two other ELISAs detected neither IgG nor IgA.
Precisely 1 year later, in April 2021, the patient presented again with fever and concomitant headache developed the previous day. He had received a dose of SCIG the day the fever had started. PCR was positive for SARS-CoV-2. Further testing revealed deletion 69/70 and N501Y, classifying the virus as the variant of concern (VOC-202012/01) or B.1.1.7, later assigned as the alpha variant (see Supplemental Table 1 for further details).
Leukocytes, lymphocytes and lactate dehydrogenase were normal, while the platelet count was slightly decreased (121 × 109/L), and the CRP was elevated (49.4 mg/L rising to 154.4 mg/L). Procalcitonin was initially slightly elevated at 0.57 µg/L (normal values: <0.50 µg/L). The patient was admitted to the hospital and received empiric antibiotic treatment, intravenous fluids, anti-pyretics and low-molecular-weight heparin. After confirming the SARS-CoV-2 infection, monoclonal SARS-CoV-2 antibodies casirivimab (1,200 mg) and imdevimab (1,200 mg) (Regeneron Pharmaceuticals, Rensselaer, NY, USA) were administered. We decided to use the monoclonal antibodies first instead of remdesivirs because we expected that they would replace the missing physiological antibody response.
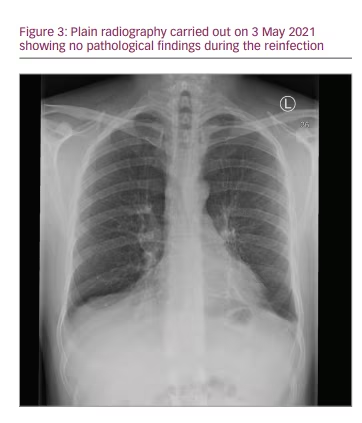
Signs of hyperinflammation, apart from the markedly elevated CRP, were mostly clinical. While the patient was in a better condition than in the first episode, he was still very sick with a high fever. The fever resolved, and no supplemental oxygen or further anti-viral medication was needed. Since low levels of bacteria, Campylobacter jejuni, were discovered in the blood cultures, the patient was kept in the hospital for further observation and intravenous antibiotic treatment for 7 days. He was discharged free of symptoms, with two negative PCR tests and no signs of infiltration on a chest X-ray at 10 days (Figure 3). Testing for antibodies again proved inconclusive. No antibodies against SARS-CoV-2 nucleocapsid protein were detected, but tests for anti-SARS-CoV-2 spike IgM and IgG were positive; confounding treatment with monoclonal anti-spike IgG1 and SCIG should be taken into account.
To rule out possibilities other than reinfection, such as prolonged viral shedding, we performed a variant screening on the stored samples from the initial infection. In this sample, no variants of concern were discovered, suggesting that it was the Wuhan strain.
Discussion
The role of neutralizing antibodies in the viral clearance and prevention of SARS-CoV-2 infection and reinfection has become a main focus since the beginning of the pandemic. Moreover, antibody concentration is of particular interest when evaluating the efficacy of vaccinations and booster campaigns. Therefore, patients with immunodeficiency have been initially regarded as at risk for a severe course of COVID-19.2 The natural course, however, and the specific benefit of the available therapies, especially convalescent plasma, in these patients are still unknown.
So far, 18 case reports and small series of a total of 32 patients with XLA contracting COVID-19 have been published.3–20 Most patients had a mild course of COVID-19 and were able to clear the virus. Initially, this suggested that antibodies may not be decisive for clearing the disease in patients with functional cytotoxic T-cells. Moreover, it was speculated that the lack of B-cell-derived cytokines, such as interleukin-6 and tumour necrosis factor-α, may prevent the ‘cytokine storm’ seen in severe COVID-19.7,8,16 Recently, reports on prolonged and severe disease courses, including one deceased patient, have been published.4,5,12,18 They show that there are indeed patients with severe inflammation. Only one patient, however, needed mechanical ventilation.12 Thirteen of these 32 patients received convalescent plasma at varying time points, often after several weeks of illness. Most patients recovered quickly thereafter; no unfavourable outcomes associated with convalescent plasma were reported.21
Even without a clear benefit being shown in randomized studies, some authors argued that, in a specific group of patients with COVID-19, such as those with primary immunodeficiencies, viral clearance may depend on neutralizing antibodies provided by convalescent plasma.22 While we decided against its administration during our patient’s first infection due to mild disease, convalescent plasma as a therapeutic option had been largely replaced by monoclonal antibodies at the time of his second infection. Anti-spike IgG1 antibodies such as imdevimab and casirivimab were broadly introduced to prevent hospitalization and severe disease in patients in the early phase of the SARS-CoV-2 infection.23 In our case, the second infection was milder and shorter than the first. We can, however, not decide about the contribution of monoclonal antibody administration to the observed mild disease course or, indeed, the role of cytotoxic T-cells or the virus variants.
Most importantly, the specific role of neutralizing antibodies and cytotoxic T-cells in clearing the virus and preventing reinfection generally and in patients with XLA particularly is unclear. Reinfection with SARS-CoV-2, first reported in the summer of 2020,24 at this time was rare given the numbers of infected patients. Clinical characteristics, duration and course of reinfections, as well as risk factors, have not been defined yet. To the best of our knowledge, the patient presented here was the first confirmed patient with XLA and SARS-CoV-2 reinfection. There is only one case report on a suspected reinfection less than 3 weeks after initial recovery in a patient with XLA. This published case does show a possible but not undoubtedly proven reinfection, given the short time between the first and presumed second episode.5 The short time, however, does not exclude reinfection. Long-term shedding, as described in several patients with XLA,12,18 or recrudescence and a second peak of illness as also described,18 could not be ruled out as no sequencing was performed. In our case, reinfection was undoubtedly confirmed by variant screening, which revealed infection with a different variant after 1 year, highlighting that these patients indeed face the risk of reinfection. Apart from specific aspects in patients with primary immunodeficiency, this case illustrates the impressive progress in diagnostics and therapy over 1 year of the COVID-19 pandemic.








